
Monmouth County, New Jersey Feb 6, 2026 (Issuewire.com) - DDi, a leading provider of regulatory and clinical automation solutions, today announced the launch of Visu UDI, a comprehensive Global UDI Compliance software designed to transform how medical device manufacturers manage regulatory data. As health authorities worldwide enforce stricter tracking standards, Visu UDI offers a strategic answer to the industrys growing data fragmentation challenge.
Moving beyond simple submission tools, Visu UDI integrates Master Data Management (MDM) directly with Regulatory Information Management (RIM) ecosystems. This "Single Source of Truth" approach allows manufacturers to pool disparate data from ERP, PLM, and labeling systems into a unified SmartMaster component.
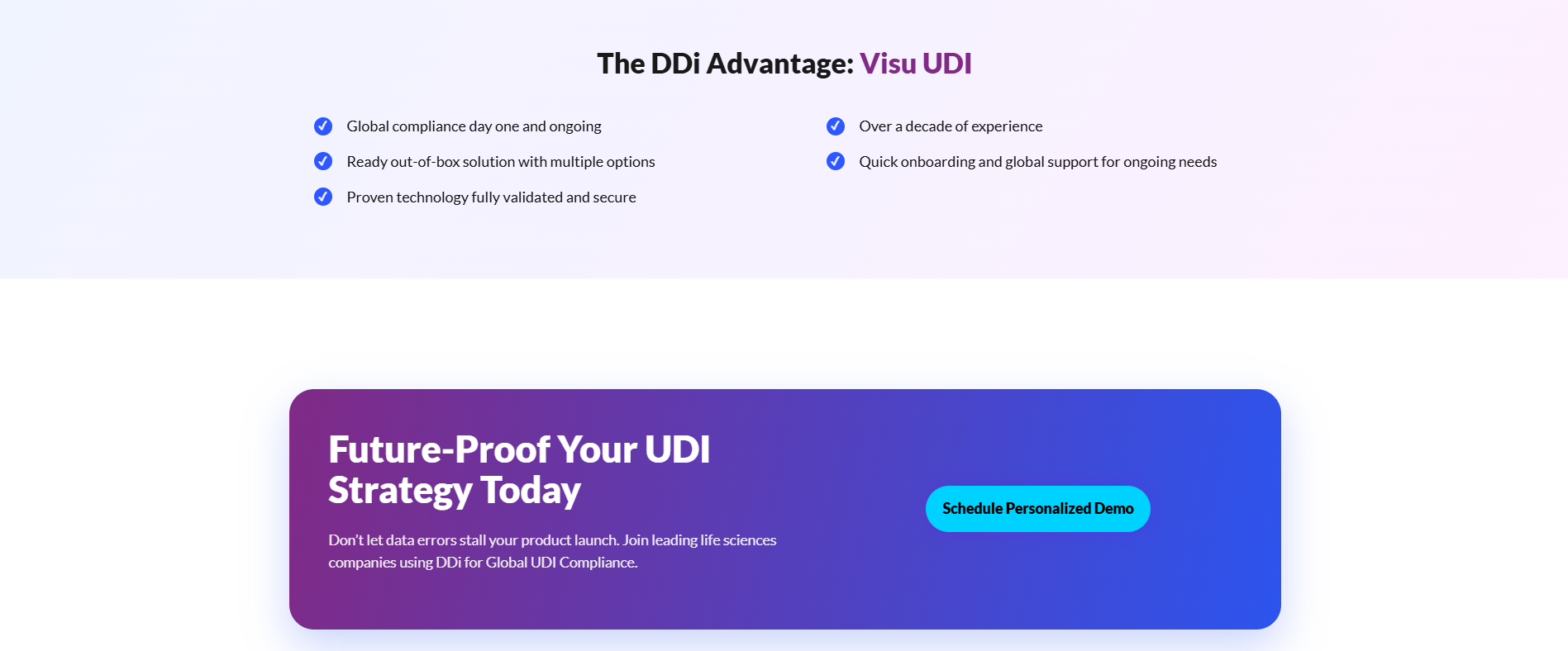
"Managing UDI requirements individually country-by-country is both cumbersome and expensive," said a DDi spokesperson. "Visu UDI centralizes this process, ensuring medical device data is accurate, compliant, and ready for global markets."
Key Features of Visu UDI include:
- Integrated MDM/RIM: Breaks down silos between regulatory, supply chain, and quality teams by linking UDI data with registration certificates and dossiers.
- Multi-Market Coverage: Comes with ready-to-use templates for FDA (USA), EUDAMED (EU), NMPA (China), TGA (Australia), and more.
- Flexible Deployment: Available in three models to suit business needs, ranging from a pay-per-use conversion tool to a fully integrated enterprise solution with proprietary smartRules engine automation.
For more information, visit https://www.ddismart.com/udi-unique-device-identification-solutions/


Media Contact
DDi Smart
More On Bestofnewsupdates ::
- Prefabricated 40ft Double-Wing Expandable House Price Trends 2025
- Safety on the World Stage: CBRNE - CT Atlantic Bridge and Sunset Bluff Form Global Events Alliance
- Sherry Parsons, Recognized by BestAgents.us as a 2025 Top Agent
- Veterans Use GROK AI to Show Veteran Service Organizations "Missing in Action" on X
- Dr. Krishna Saksena’s new book titled ‘Flowers Bloom’ unveiled in New Delhi by Dr. Kalyan Banerjee
(877) 877-1519
Princeton Park Corp Center, 1100 Cornwall Road, Suite 110
Source :DDi Smart
This article was originally published by IssueWire. Read the original article here.
16 day's ago